Kemvia, the first-ever AI-integrated CRDMO platform that seamlessly connects Innovators and CRDMO Partners through a unified digital ecosystem that bridges Discovery, Development, and manufacturing. Across small molecules, biologics, ADCs, AOCs, cell & gene therapies and other emerging drug modalities, Kemvia empowers faster, smarter molecule-to-market transitions — transforming innovation into impact.
Trusted By
Explore Our Solutions
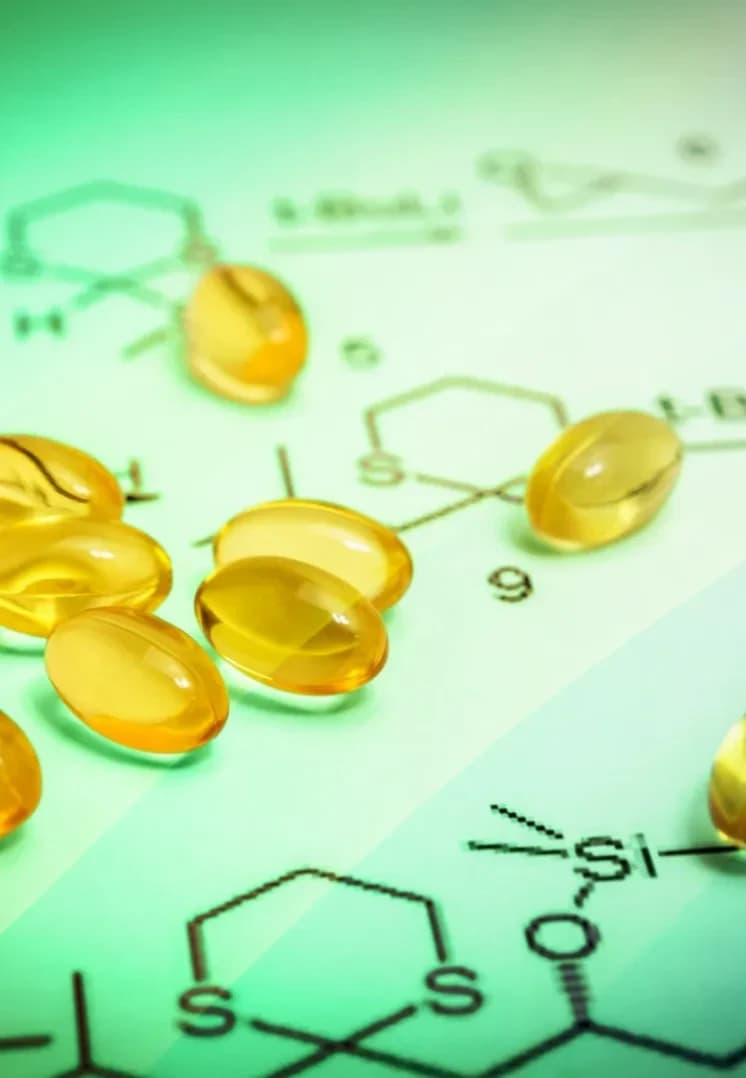
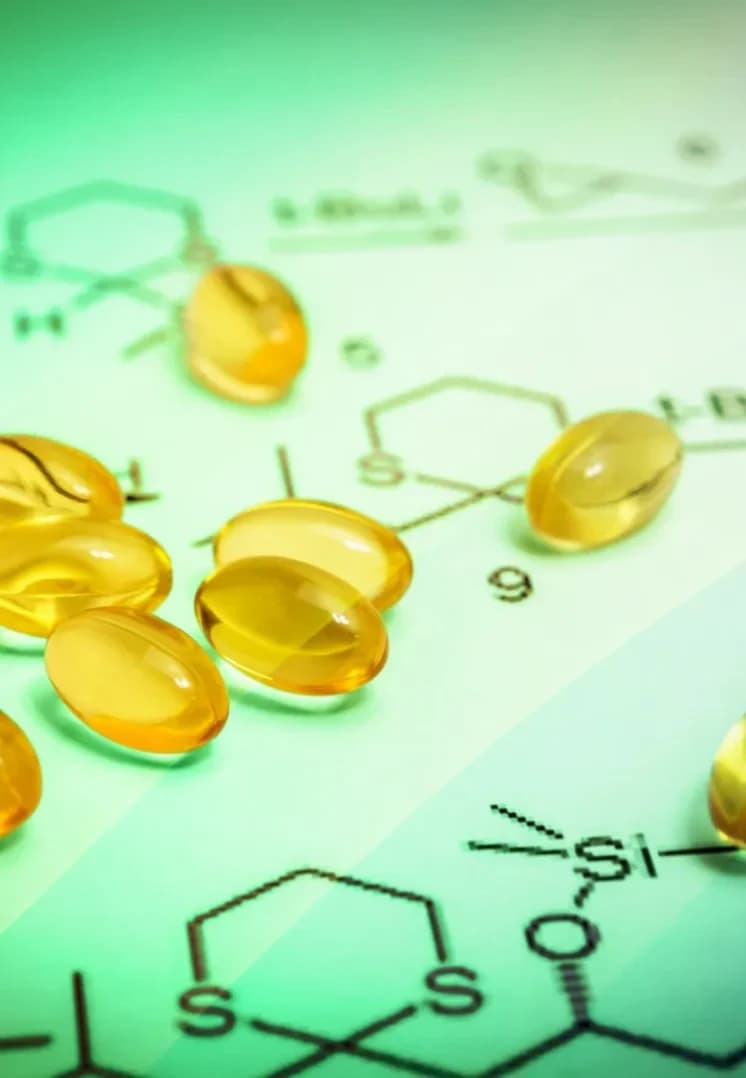
Small Molecules
Small molecule drugs are the workhorses of modern medicine. Defined by a low molecular weight—typically under 900 Daltons - these compoundsare chemically optimized for efficiency. Their compact structure allows them to rapidly navigate the gastrointestinal tract, enter the bloodstream, and easily penetrate cellmembranes to reach their targets. This superior bioavailability explains why small molecules dominate the landscape, accounting for an estimated 90% of all drugs on themarket. Beyond their biological efficiency, they offer a critical practical advantage: they can be administered orally, providing unmatched convenience for patients of allages.
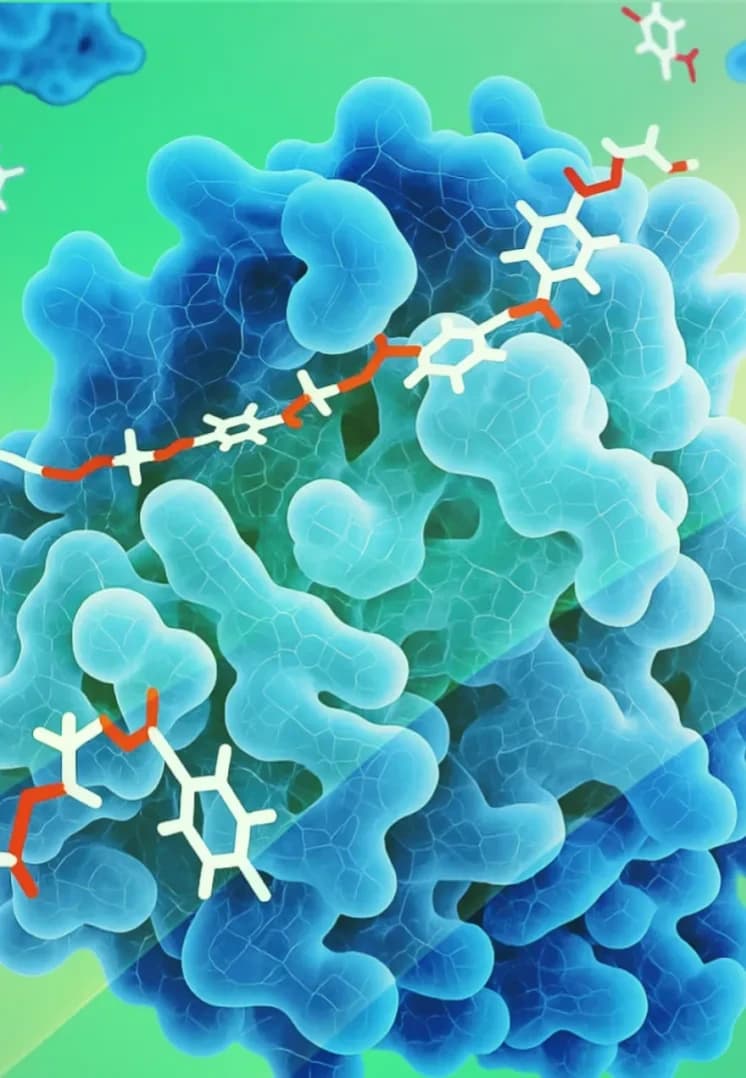
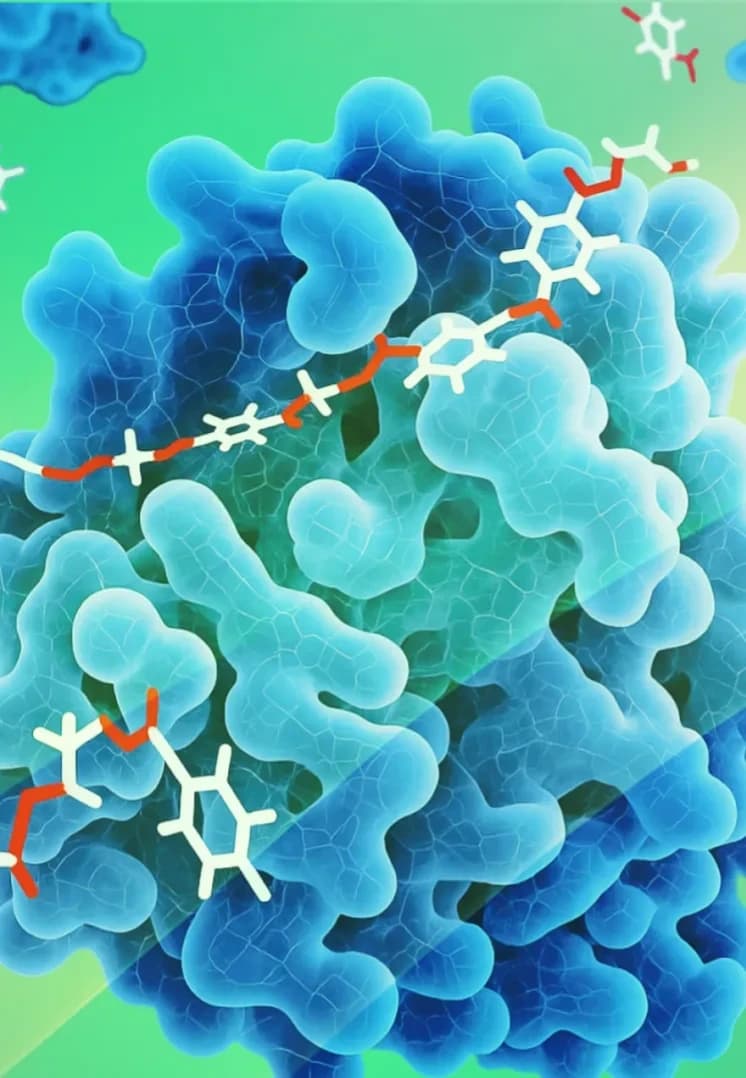
Proteins
These are chains of amino acids that can be engineered to have specific biological activities, such as binding to receptors or modulating cellular processes.
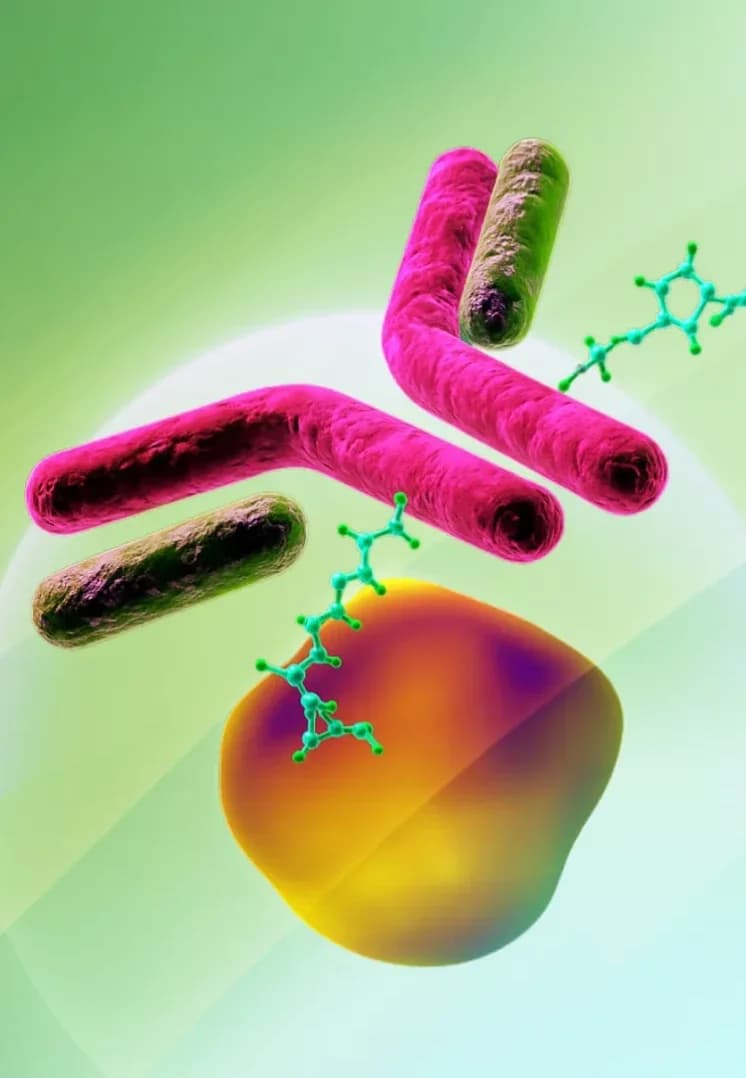
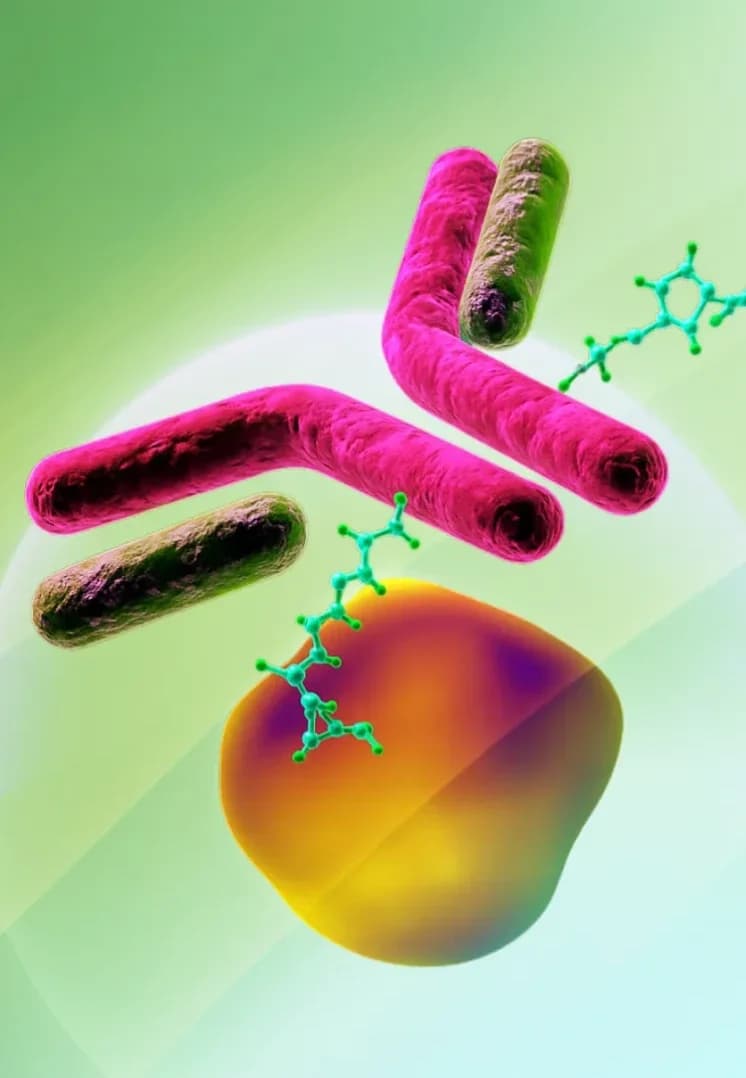
Antibody Drug Conjugates
ADCs combine the targeting ability of antibodies with the therapeutic payload of a small molecule, allowing for targeted drug delivery.
Antibody Oligonucleotide Conjugates
Lorem Ipsum is simply dummy text of the printing and typesetting industry.
Small Molecules
Proteins
Antibody Drug Conjugates
Antibody Oligonucleotide Conjugates
What is kemvia?
Your Molecule. Our Platform. One Seamless Journey.
Our platform is more than a marketplace - it's a decision-support engine & an operational backbone. Designed to reduce fragmentation & manual inefficiencies, it enables pharma innovators to plan, develop, source, manufacture & scale molecules faster & smarter.
Your Molecule. Our Platform. One Seamless Journey.
Our platform is more than a marketplace - it's a decision-support engine & an operational backbone. Designed to reduce fragmentation & manual inefficiencies, it enables pharma innovators to plan, develop, source, manufacture & scale molecules faster & smarter.
End-to-End Lifecycle Coverage
From discovery to post-commercialization harmonized operations, tech transfer, and regulatory integration in one platform.
Al-Driven Speed & Precision
Predictive algorithms, intelligent matchmaking, and adaptive workflows ensure faster and smarter decisions.
Global Partner Ecosystem
A curated network of GMP-compliant CDMOs, CROs, suppliers, and technology partners across modalities and geographies.
Capabilities That Drive Innovation
Drug Discovery & Preclinical Enablement
The early stages of drug development and manufacturing involve research, testing, and evaluation of potential molecules, and it sets the stage for the entire project’s success.

Process Development & Scale-Up
Advanced process development and scale-up solutions to accelerate your drug development timeline with proven methodologies and expert guidance.

Clinical & Commercial Manufacturing
End-to-end manufacturing capabilities from clinical trials to commercial production with full regulatory compliance and quality assurance.

Regulatory Intelligence & Compliance
Advanced process development and scale-up solutions to accelerate your drug development timeline with proven methodologies and expert guidance.
Post-Market Lifecycle Services
Advanced process development and scale-up solutions to accelerate your drug development timeline with proven methodologies and expert guidance.


RFP Win Rate Over the
last 1 year
Orderbook for FY26
(in INR Mn)
Total CRDMO Clients
(API + Formulations)